Our Vision:
We are an emerging start-up, aspiring to offer new perspectives for patients with pancreatic, liver and bile-duct cancers. Ultimately, aiming for patients to live longer, healthier, and more productive lives.
Our Mission:
Clearing tumors from blood vessels, so surgeons can operate.
What we do:.
We develop VasoBLATE™ – an innovative perivascular ablation solution.

Our Solution – VasoBLATE™
That is why we are developing VasoBLATE™, an innovative perivascular ablation solution aimed at creating clean margins around blood vessels, clearing the way for surgeons to operate and ultimately improving the patient’s chances for survival.
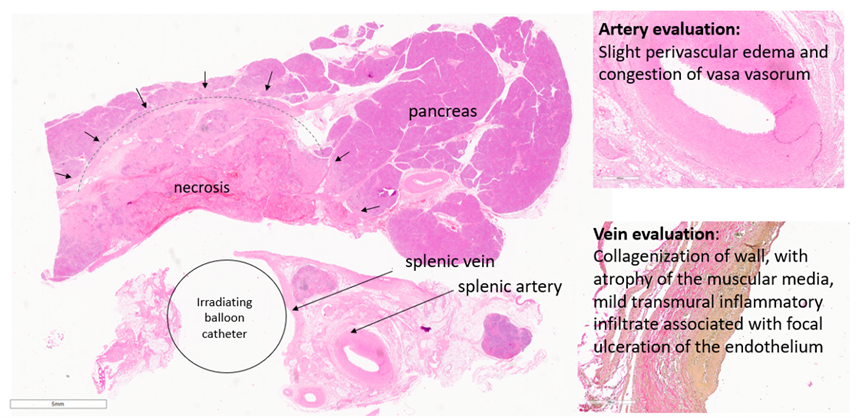
VasoBLATE is based on the administration of a laser-light activatable drug that accumulates in the tumor tissues. The drug is then activated by laser light to locally destroy the tumor tissues.

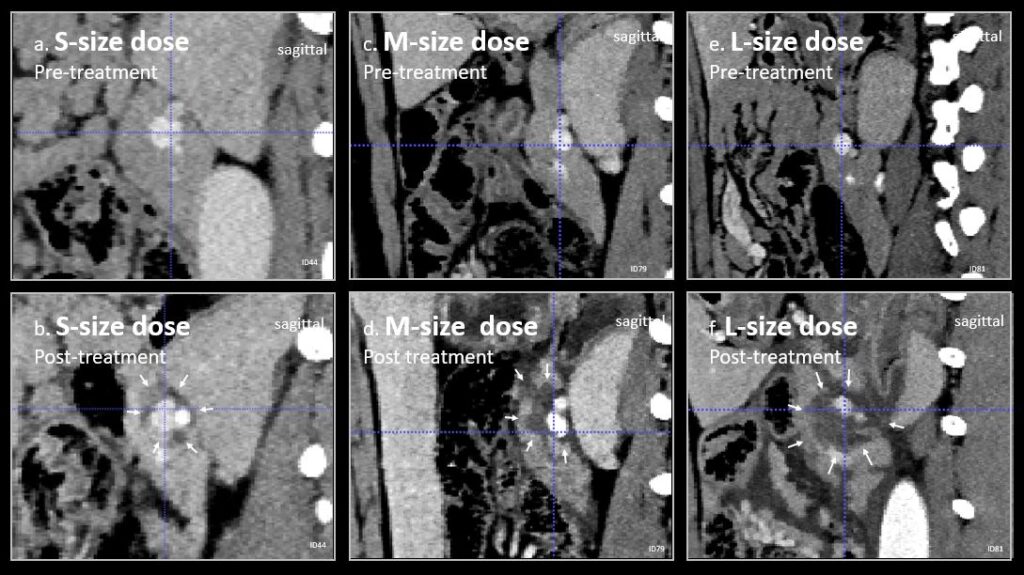
Minimally-invasive, image-guided therapy
Minimally-invasive, and under image-guidance, a near-infrared laser-light irradiating catheter is placed in the target vessel encased by tumor tissue. The near-infrared laser light then travels though the vascular wall, activates the drug, and locally destroys the tumor tissues, paving the way for surgeons to operate.
VasoBLATE™, comprises:
- PeriPLAN™ – Treatment planning & verification software
- PeriLASE™ – A near infra-red laser
- PeriPORFIN™ – A light-activatable drug
- PeriCATH™ – A near-infrared laser-light irradiating balloon catheter

PeriPLAN™
Treatment planning & verification software

PeriLASE™
Near-infrared laser

PeriPORFIN™
Laser-activatable drug

PeriCATH™
Laser-irradiating balloon catheter

VasoBLATE™
Perivascular ablation solution


Team
The company is managed by a passionate team of three senior founders (CSO, CMO, CEO). Our team has several decades of combined medical, scientific, and business experience with the development of drug-device combination products such as these and have direct experience taking these through Phase I/II clinical studies.

Investment need
We are currently seeking a seed investment to initiate the Phase I clinical study and begin treating the first patients.
This significant milestone will transform and mature our company from a pre-clinical to a clinic-stage start-up.
Our transformation to the clinic will substantially increase our company valuation and enhance our chances of attracting future investors for next funding rounds. In line with this goal, we have already started discussions with Angels, Family Offices, and Venture Capital firms.
For Angel investors we offer Convertible Notes and SAFEs at attractive Terms & Conditions.
Sign-up
Please sign-up if you would like to stay informed about our latest news & developments.
Disclaimer:
The VasoBLATE™ perivascular ablation solution described herein is under investigation by research groups in preclinical experiments. It is currently not regulatory approved in any jurisdictions.
Vascular Oncology
Biotechnologies B.V.
© 2026